19.1 Hebb rule and experiments
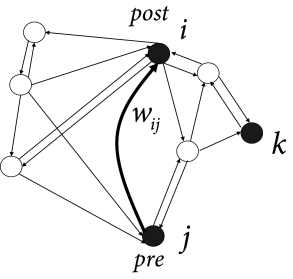
Since the 1970’s, a large body of experimental results on synaptic plasticity has been accumulated. Many of these experiments are inspired by Hebb’s postulate ( 210 ) that describes how the connection from presynaptic neuron to a postsynaptic neuron should be modified:
When an axon of cell is near enough to excite cell and repeatedly or persistently takes part in firing it, some growth process or metabolic change takes place in one or both cells such that ’s efficiency, as one of the cells firing , is increased.
| A | B |
|---|---|
|
|
Today this famous postulate is often rephrased in the sense that modifications of the synaptic transmission efficacy are driven by correlations in the firing activity of pre- and postsynaptic neurons; cf. Fig. 19.1 A. The shortest summary is: neurons that ‘fire together, wire together’ ( 471 ) . Note that the term ‘fire together’ is less precise than Hebb’s original formulation which contains an asymmetry since a neuron that ’contributes to firing’ another one has to be active slightly before the latter. Even though the idea of learning through correlations dates further back in the past ( 242 ) , correlation-based learning is now generally called Hebbian learning .
Hebb formulated his principle on purely theoretical grounds. He realized that such a mechanism would help to stabilize specific neuronal activity patterns in the brain; cf. Fig. 19.1 B. If neuronal activity patterns correspond to behavior, then stabilization of specific patterns implies learning of specific types of behaviors ( 210 ) . We emphasize that Hebbian learning is unsupervised, because there is no notion of ‘good’ or ‘bad’ changes of a synapse. Synaptic changes happen whenever there is joint activity of pre- and postsynaptic neurons, i.e., they are driven by the neuronal firing patterns. These patterns may reflect sensory stimulation as well as ongoing brain activity, but there is no feedback signal from a ‘supervisor’ or from the environment.
In this section we review experimental protocols that induce lasting synaptic changes and discuss their relation to Hebbian learning.
19.1.1 Long-Term Potentiation
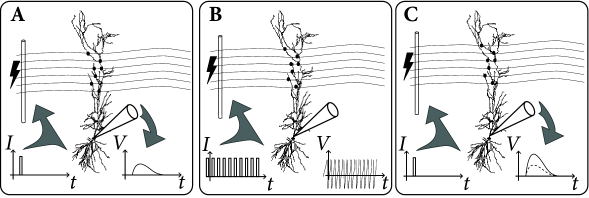
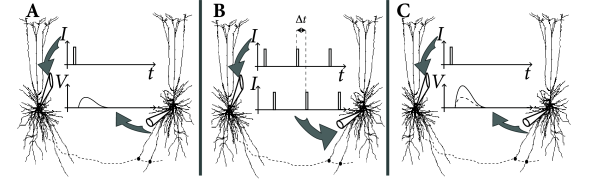
The classic paradigm of LTP induction is, very schematically, the following ( 75; 60 ) . Neuronal activity is monitored by an extracellular or intracellular electrode, while presynaptic fibers are stimulated by means of a second (extracellular) electrode. Small pulses are applied to the presynaptic fibers in order to measure the strength of the postsynaptic response (Fig. 19.2 A). The amplitude of the test pulse is chosen such that the stimulation evokes a postsynaptic potential, but no action potentials.
|
| D |
|
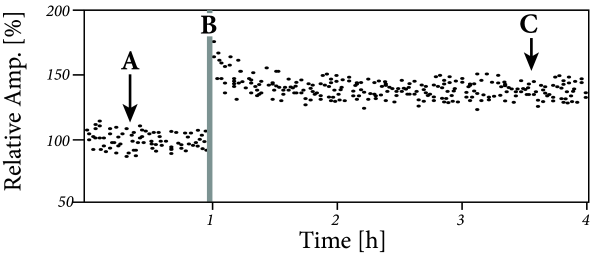
In a second step, the input fibers are strongly stimulated by a sequence of high frequency pulses so as to evoke postsynaptic firing (Fig. 19.2 B). After that, the strength of the postsynaptic response to small pulses is tested again and a significantly increased amplitude of postsynaptic potentials is found (Fig. 19.2 C). This change in the synaptic strength persists over many hours and is thus called Long-Term Potentiation or LTP (Fig. 19.2 D).
The increase of the synaptic weights can be interpreted as ‘Hebbian’, because it occurred after an episode of joint activity of pre- and postsynaptic neurons. Early LTP experiments were done with two extracellular electrodes (one for the stimulation of presynaptic fibers, the other for the measurement of the neuronal response), but in later experiments LTP was also studied with intracellular recordings.
Example: Voltage dependence of LTP
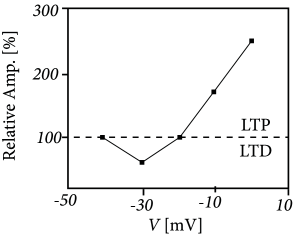
With an intracellular electrode, an experimentalist cannot only record the response to an incoming spike, but can also manipulate the membrane potential of the postsynaptic neuron; cf. Fig. 19.3 . If presynaptic spikes arrive during a period where the neuron is strongly depolarized, LTP is induced at the activated synapses. On the other hand, spike arrival combined with weak depolarization causes LTD ( 29; 30; 365 ) . These and similar experiments reveal the importance of the postsynaptic voltage during the induction of synaptic plasticity. If we interpret strong depolarization of the postsynaptic neuron as a substitute of neuronal activity, the above protocol for LTP induction can be called ‘Hebbian’.
19.1.2 Spike-timing-dependent plasticity
Pairing experiments with multiple intracellular electrodes in synaptically coupled neurons have opened the possibility to study synaptic plasticity at an excellent spatial and temporal resolution ( 328; 567; 117; 55; 56; 483 ) ; see ( 54; 482 ) for reviews.
|
||
| D | E | F |
|
|
|
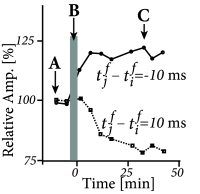
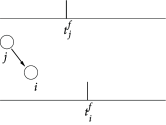
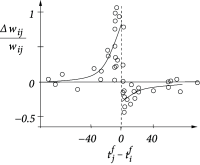
Figure 19.4 illustrates a pairing experiment with cultured hippocampal neurons where the presynaptic neuron ( ) and the postsynaptic neuron ( ) are forced to fire spikes at time and , respectively ( 55 ) . The resulting change in the synaptic efficacy after several repetitions of the experiment turns out to be a function of the difference between the firing times of pre- and postsynaptic neuron. This observation has given rise to the term ‘Spike-Timing-Dependent Plasticity’ (STDP). Most notably, the direction of the change depends critically, on the relative timing of pre- and postsynaptic spikes on a millisecond time-scale ( 328 ) . The synapse is strengthened if the presynaptic spike occurs shortly before the postsynaptic neuron fires, but the synapse is weakened if the sequence of spikes is reversed; cf. Fig. 19.4 B. This observation is indeed in agreement with Hebb’s postulate because presynaptic neurons that are active slightly before the postsynaptic neuron are those which ‘take part in firing it’ whereas those that fire later obviously did not contribute to the postsynaptic action potential. An asymmetric learning window such as the one in Fig. 19.4 F, is thus an implementation of the causality requirement that is implicit in Hebb’s principle.
Similar results on spike-timing-dependent plasticity have been found in various neuronal systems ( 3; 53; 89 ) , but there are also characteristic differences. Synapses between parallel fibers and ‘Purkinje-cells’ in the cerebellar-like structure of electric fish, for example, show the opposite dependence on the relative timing of presynaptic input and the (so-called ‘broad’) postsynaptic spike ( 43 ) . In this case the synapse is weakened if the presynaptic input arrives shortly before the postsynaptic spike (anti-Hebbian plasticity).